Breaking News – Health Canada has approved the use of a portable, rapid-testing device for COVID-19.
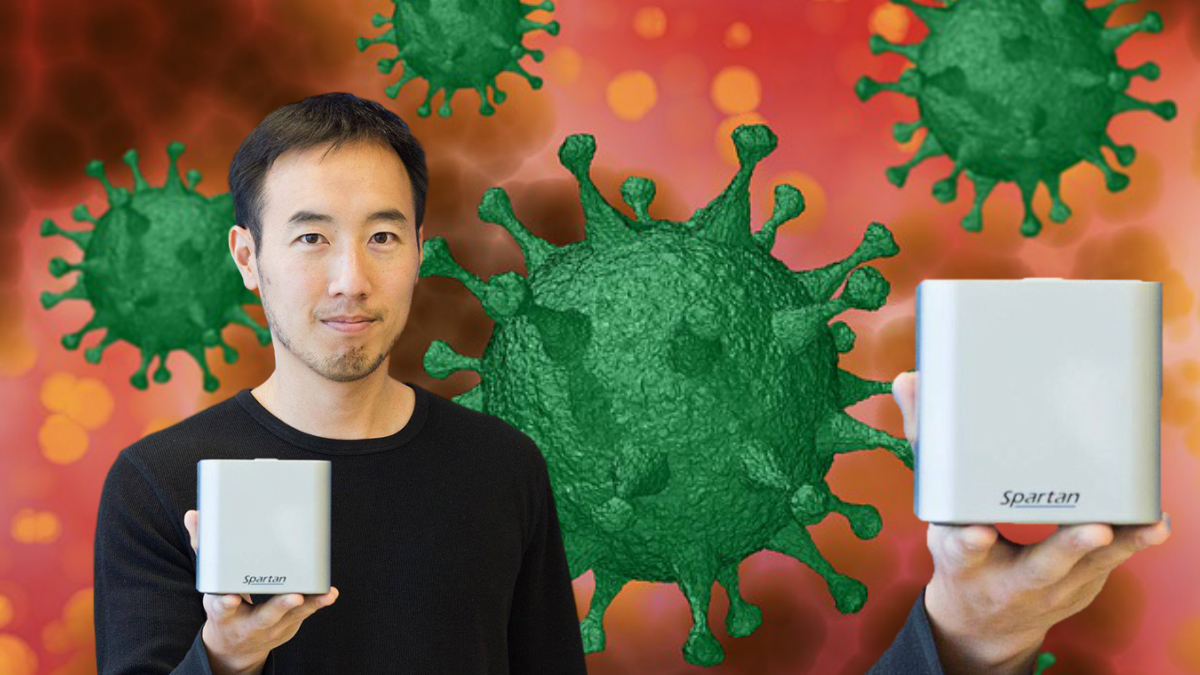
The Spartan Cube from Ottawa’s Spartan Bioscience will help provide rapid tests for health services in rural and remote areas such as Indigenous communities.
The hand-held device, about the size of a coffee cup, eliminates the need for swab samples to travel to the nearest lab, which can be a logistical challenge.
The federal, Alberta, and Ontario governments are among those who have contracts for the testing kits, which can confirm results in less than one hour.

Ontario has ordered nearly 1 million testing kits, while Alberta’s contract is for 100,000 testing kits. The Cube uses Spartan’s COVID-19 test cartridges and proprietary swabs, manufactured in Ottawa.
Health Canada greenlit the device on Saturday, and Spartan says it will begin shipments “immediately.”
Spartan says the test, in which either the nose or throat is swabbed, can be operated by non-laboratory personnel in a variety of locales such as airports, border crossings, doctors’ offices, pharmacies, and clinics.
“There is an urgent unmet need for rapid COVID-19 testing, and as a proudly Canadian company, we are excited that our technology will be an important part of fighting the COVID-19 pandemic in Canada,” CEO Paul Lem said Monday in a release.
This report by The Canadian Press, first published on April 13, 2020.
The Canadian Press